A breakthrough is taking place in the world of science and technology that has the potential to radically change our understanding and use of hydrogen. A research team consisting of experts from Charles University, IT4Innovations at VŠB - Technical University Ostrava and the Czech Academy of Sciences has achieved significant results in the study of the interaction of actinides with hydrogen. These findings were recently published in the prestigious journal Reports on Progress in Physics, representing a significant milestone in the field of research.
Ladislav Havela from Charles University, Dominik Legut from IT4Innovations and Jindřich Kolorenč from the Czech Academy of Sciences focused on investigating the physical properties of actinide hydrides and their interaction with hydrogen. This interest stems mainly from the need to better understand the processes that take place when hydrogen enters metals, which can have both positive and negative consequences.
One of the key discoveries is that uranium and plutonium in hydride form exhibit ferromagnetism, a property that usually only occurs at supercritical distances between the two nearest uranium atoms. This discovery offers new opportunities for research and practical applications of these materials.
The research also revealed that actinide compounds with hydrogen, such as LaH10, exhibit superconductivity at temperatures as low as 260 K, but at extremely high pressures, amounting to 180 GPa. This discovery opens the door to further exploration of the role of hydrogen and actinides in the mechanism of superconductivity.
Interestingly, the research is not limited to theoretical findings. Researchers are also trying to apply their findings in practice, for example in the field of nuclear fusion, where uranium metal can be used to store and release tritium, a key isotope for fusion reactions.
The study also shows that inserting hydrogen atoms into the interstices of the uranium crystal lattice leads to its expansion and charge transfer, which could have important implications for the development of new materials and technologies.
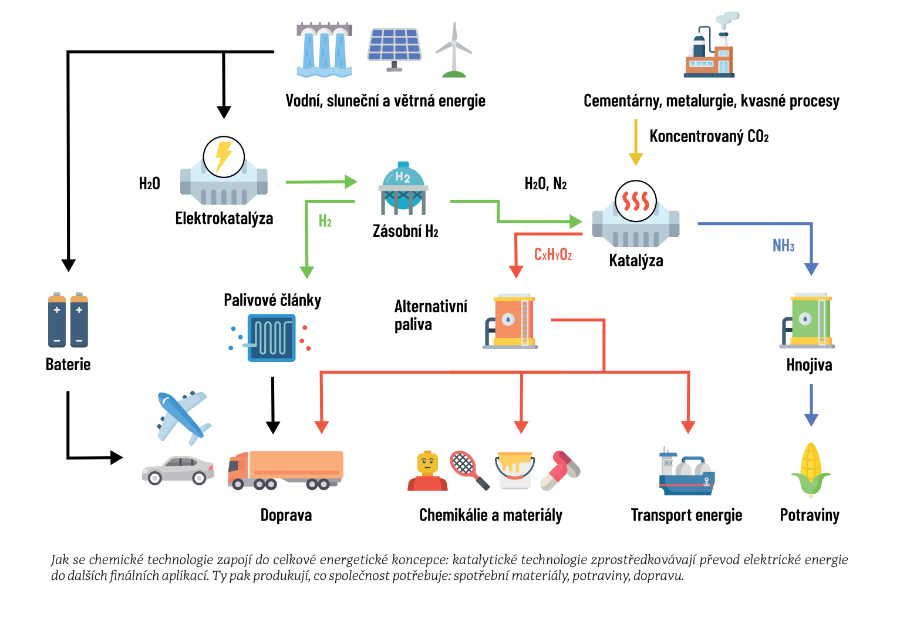
Understanding the interaction between actinides and hydrogen can have broad applications, including the development of better storage systems for hydrogen, which is key to its use as a clean energy source. Hydrogen, long seen as a promising but technically challenging energy source, could thus be a step closer to wider use.
In addition, the research is contributing to an understanding of fundamental physical processes that could lead to new discoveries in a range of other fields, from materials science to chemistry and energy.
The team of scientists is at the forefront of research that could revolutionise energy and materials science. Their work highlights the importance of collaboration between academic institutions and the potential that hydrogen as an element offers for the future.
This research not only represents a significant contribution to the scientific community, but also promises to lay the groundwork for innovations that could fundamentally change our energy systems and contribute to environmental protection. Scientists and engineers now face the challenge of translating these findings into practical applications that could lead to greater efficiency, safety and sustainability in the energy sector.
Graphic and text source: https://www.avcr.cz/export/sites/avcr.cz/cs/veda-a-vyzkum/avex/files/2024-01.pdf
Photo generated by AI